TL;DR Science: Patterns in the Periodic Table - Introductory Chemistry
By Thomas P.
October 21, 2022 · 3 minute read
Chemistry
Physics
The periodic table is a way of organizing the various chemical elements. As you may or may not know, the table is organized by the number of protons and electrons in the atom (known as the atomic number) and its average atomic mass. However, what if I told you that patterns existed within the periodic table beyond just succeeding atomic number after number? These patterns occur periodically and predictably throughout the table, which makes the arrangement so ingenious.
Parts of the periodic table
The periodic table, similar to other tables, is split up into rows and columns. A column is called a group because elements within are going to have similar properties, and a row is called a period, because properties will periodically change as one hops from group to group.
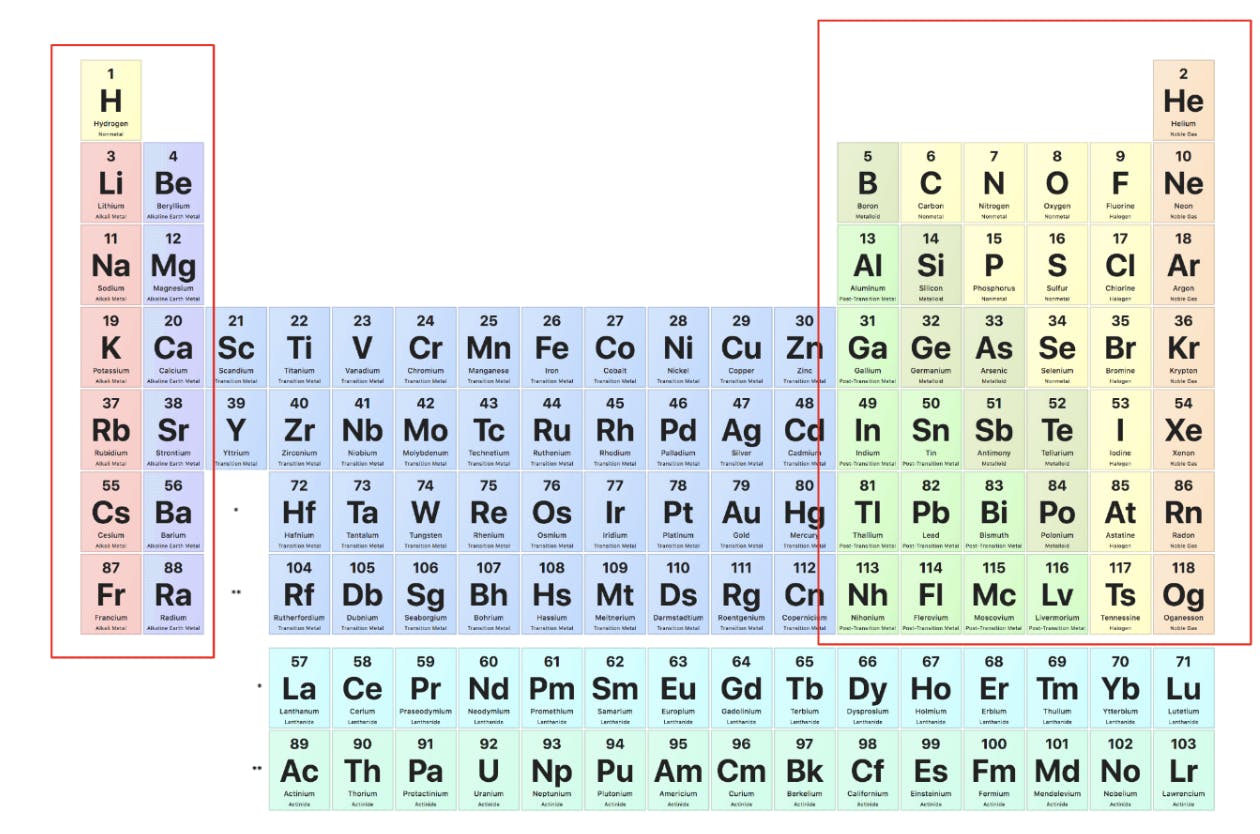
The elements boxed in red above are called the representative elements because they have similar properties regarding valence electrons (electrons on the outer rings of an atom). The elements not boxed in red are the transition elements, because they do not share these properties.
The Atom and Chemical Reactions
Before we talk about the periodic table, it is necessary to briefly discuss the nature of the atom, since many principles of the periodic table are based on its structure and the electrons surrounding it.
An atom is made up of a nucleus and an electron shell. The nucleus is made up of a certain number of protons that corresponds to the element’s atomic number (e.g. Hydrogen is 1) and a certain number of neutrons that depends on what type of isotope the element is. The number of neutrons within the nucleus can be determined by subtracting the isotope’s atomic mass from the atomic number. For example, Carbon-13 has an atomic mass of 13 amu (atomic mass units); its mass number is 6. Therefore, it has 7 neutrons. Remember that protons have a positive charge and neutrons a neutral one; this positive charge makes it attract to the negatively charged electron
The electron shell is made up of a certain number of electrons that corresponds to an element's atomic number. For all representative elements (excluding Hydrogen and Helium), the element is not stable unless it can obtain an outer shell (“valence shell”) of 8 electrons. This means that the element’s life goal is to by any means possible (including stealing or giving away one or more of its outer electrons when reacting with another element).
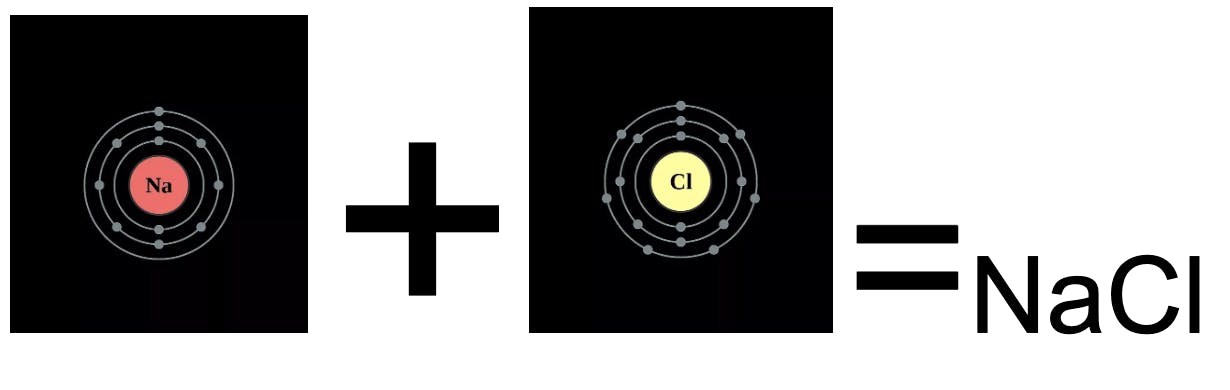
(Needs to lose 1 valence electron) + (Needs to gain 1 valence electron) → (stable)
Valency in Compounds
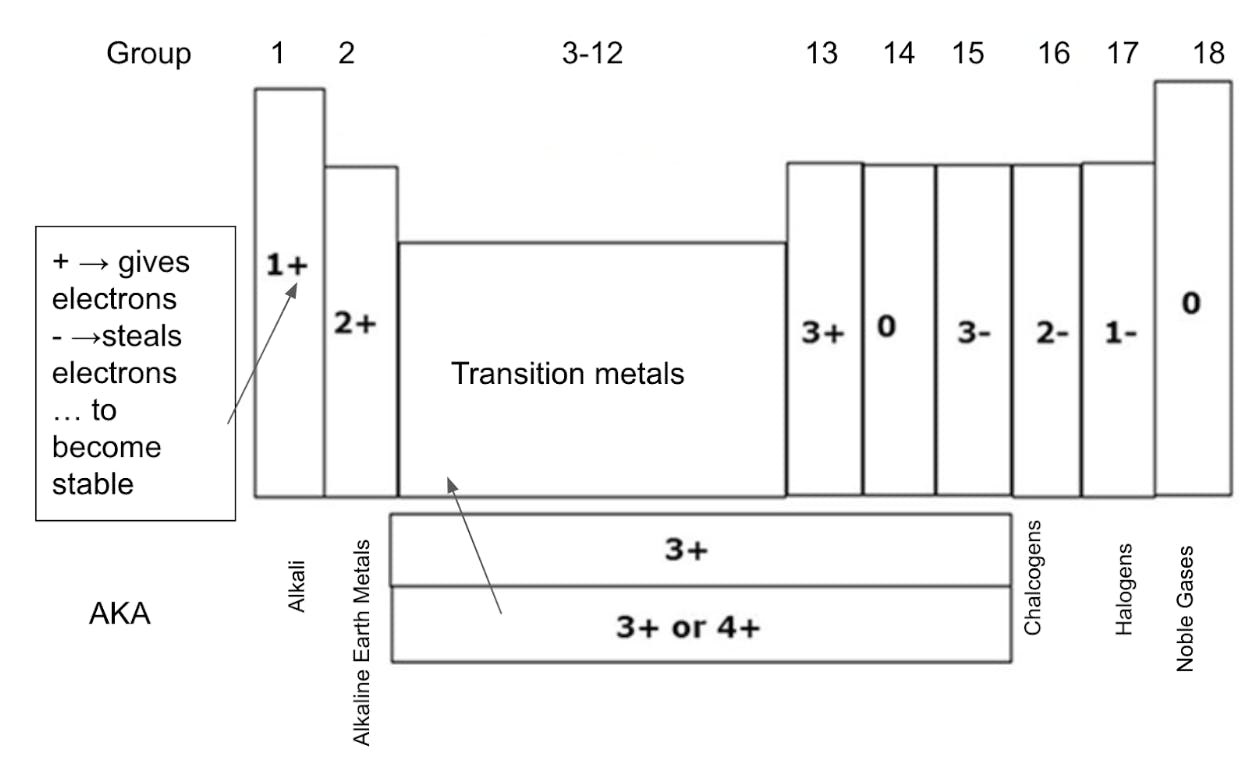
As described above, the number of valence electrons in a representative element determines how reactive an element is. Since the representative elements only includes Groups 1, 2, and 13-18, we can determine how reactive an element is based on how many electrons it needs to lose or needs to gain to become stable. For example, elements in Group 1(1+) and 2 (2+) as well as Groups 16 (2-) and 17 (1-) are more reactive than Groups 14 and 18 (0).
Note: 1+ = Must lose one electron to become stable, 1 - = Must gain one electron to become stable, 0 = Stable, unreactive
Atomic Radius
Another pattern that is visible in the periodic table is decreasing atomic radius moving across the period, and increasing atomic radius moving down the group. This phenomenon exists when moving across the period because there is an increase in the number of electrons in the outermost shell, and thus an increase in attraction between the positively-charged nucleus (containing a certain number of positively charged protons and neutrally charged neutrons) and negatively-charged electron shell. When moving down in any certain group, however, the number of electron shells increases, and therefore the distance between the outermost shell and the nucleus.
Did you enjoy this article?
About The Author
Thomas is a student at Eastside High School.