TL;DR Science: Nuclear Energy
By Eduard Shkulipa
August 18, 2021 · 3 minute read
Chemistry
Physics
Engineering
How do people create electricity?
As science has advanced, we have discovered new ways of creating energy. However, this idea of 'creating energy' is actually misleading. Due to the law of conservation of energy, energy cannot be created or destroyed. When we say that we are creating energy, we are really transforming energy from one form to another, usually to forms that are easily usable like electricity. One example of this is nuclear energy.
How do atoms create energy?
First of all, referring to the same law of conservation of energy, atoms cannot magically summon some energy. Instead, they act as accumulators. Each core of an atom consists of neutrons and protons, and the bond between these particles requires energy. This is the nuclear energy that people harvest and transform. Not all atomic bonds are made equal. In general, the more nuclei there are in the atom, the easier it is to break the atomic bonds and release the energy. This is because as the nucleus of the atom gets larger, the strong nuclear force gets weaker. Think of the strong force that holds atoms together like a planet’s gravity. As we get farther from the planet, the gravitational pull gets weaker, likewise is true for atoms. This is why heavier atoms like Uranium 235 are commonly used in nuclear reactors as their large size means that it is relatively easier for the bonds within the nucleus to be broken. Within the core of a reactor, the process of nuclear fission takes place. Nuclear fission is what we call when the nucleus of an atom splits, releasing the energy held within its bonds. Now, you can’t exactly take a knife and split an atom, so the way that nuclear reactors cause fission reactions is by shooting free neutrons (neutrons not attached to a nucleus) into atoms of U-235. As these neutrons shoot through the nucleus of the Uranium molecule, it rips the nucleus apart, releasing energy and creating more free neutrons to continue the reaction.
Harvesting and transforming Atomic Energy?
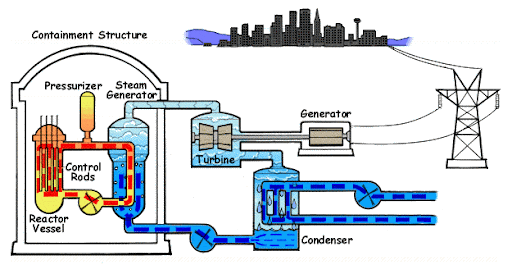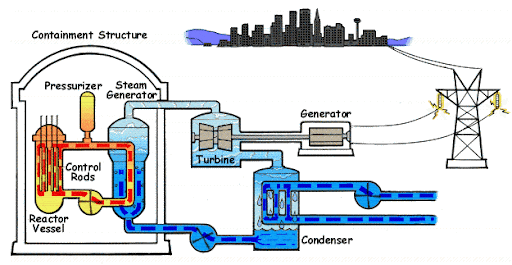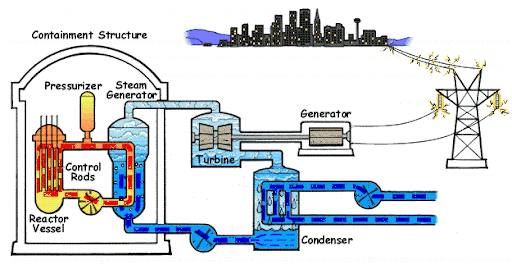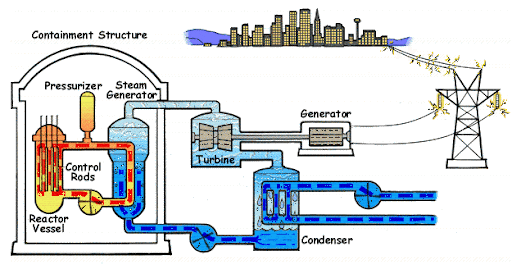
The purpose of a nuclear reactor is precisely to catch and transform the energy of nuclear fission. Nuclear power plants have different designs and they use different radioactive elements, however, the structure is common for all of them. A nuclear reactor consists of a reactor, steam generator, turbine, generator, and condenser.
- The reactor is the main component of a power plant. Inside the reactor are radioactive elements. In older versions of reactors, there is Uranium 235, however, in newer versions, Plutonium 238 is more common as a fuel. During fission, which we discussed earlier, the energy of bonds is freed into the environment. The reactor is filled with water which catches this energy and heats.
- The reactor is connected to the first circuit of water flow with a pump to move the water in a circuit. A pressurizer controls the pressure inside the reactor so it does not blow up, and safety rods that are located right above the reactor that control the speed of nuclear fission so the nuclear reaction will not get out of control. Even with all these controls, nuclear fission is so powerful that the average temperature of the water inside this circuit is 600° Celsius or 1100° Fahrenheit with pressure over 15-atmosphere pressures.
- The steam generator consumes the energy of the water from the first circuit and the water in the generator transforms into steam. The steam generator is simply a huge tank filled with water that is connected to another separated circuit of water flow. After transforming into vapor, the water goes to the turbine, which spins at enormous speeds (on average the turbine makes 3600 rotations per minute or 600 rotations per second!) This torque is transmitted to the generator which transforms mechanical energy (rotations) to electrical energy.
- After going through the turbine, the steam additionally goes through the condenser which condenses the steam back to the water, and the process repeats for decades.
How popular are nuclear reactors?
Despite the media coverage regarding nuclear meltdowns (Chernobyl, Fukushima), these accidents are far and between. In fact, nuclear energy is the most environmentally friendly and safe source of electricity among non-renewable sources of electricity. One reactor produces over 1 billion watts throughout the lifetime of one power plant. It is a lot of power. To compare, this much power would be enough to power 110 billion lightbulbs for a year.
Only in the United States, there are 60 nuclear power plants with a total of 98 reactors. Overall there are 443 reactors all over the world that add up to 20% of the global production of electricity. Even though many people are afraid of nuclear energy, it is greatly due to unawareness of the process and sources of energy. However, nuclear power plants are more and more common not only on our planet, but on Mars, and other outer space objects. But that is a topic for another time.
TL;DR
Nuclear energy is one of the most common sources of electricity. Atoms that make up the world around you contain huge deposits of energy within their bonds. When these bonds are broken, the energy is released. Nuclear power plants use the energy produced from breaking these atomic bonds (in a process called fission) to produce electricity.
Sources:
-https://www.enec.gov.ae/discover/how-nuclear-energy-works/
-https://www.youtube.com/watch?v=1U6Nzcv9Vws&ab_channel=Elearnin
-https://www.world-nuclear.org/nuclear-essentials/how-does-a-nuclear-reactor-work.aspx
-https://www.eia.gov/energyexplained/nuclear/nuclear-power-plants.php
-https://www.energy.gov/ne/articles/nuclear-101-how-does-nuclear-reactor-work
-https://www.wikiwand.com/en/Nuclear_power_plant
Did you enjoy this article?
About The Author
Eduard is a freshman at University of California San Diego. He enjoys engineering, math, solving Rubick’s cubes, swimming, and, strangely enough, collecting coins. Send him a question or suggestion at eduard@sciteens.org.